Check Process
Regulations
- Cosmetic Regulations
- 화장품관련 법률사항
Prohibited and Restricted Ingredients
- Ingredient Hotlist
- 화장품 제한/금지성분
- Hotlists must be supported their safety by a document or its safety regulation
- INCL
- International Nomenclature of Cosmetic Ingredient
- Safety of Cosmetic Ingredients
Cosmetic Notification Form
- Form
- 1 ~7 weeks for response
- 10 days before initial sales
- How to complete a CNF
Cosmetic Ingredient Labelling
- Guide
- 화장품 라벨링 가이드
- English & French
- Industry Guide for the labelling of cosmetics
- Labelling Requirements for Cosmetics in Pressurized Containers
- Consumer Packaging and Labelling Regulations
- examples: Not to be OTC (Over the Counter) drug
- Skin Brightening
- NOT: Skin Whitening (Racism)
- Improvement
- NOT: Cure
- Skin Brightening
Cosmetic Number
- CN is a 7-digit unique identifier assigned by Health Canada.
- Once the CNF is processed, a CN will be assigned and sent to the notifier via email.
- Attached to its package
Advanced notice of importation process for cosmetics and drugs
The advance notice of importation process applies to cosmetics and drugs that need to be modified or relabelled to be sold in Canada.
On this page:
Background
Health Canada’s evaluation of the advance notice of importation pilot process for cosmetics and drugs is complete. Based on the evaluation, this process will continue. There will be no changes to the current process and requirements, including the 3-month blanket period. We have updated and replaced the previous version of the advance notice of importation form with a PDF version.
Contact us for the latest advance notice of importation form.
Importing non-compliant cosmetics and drugs (including health products) for the purpose of sale is prohibited under the Cosmetic Regulations (CR) and Food and Drug Regulations (FDR). However, both of these regulations allow for non-compliant cosmetics and drugs to be imported under 2 conditions:
- the importer provides advance notice to a Health Canada inspector of the proposed importation
- the product is relabelled or modified as required for its lawful sale in Canada
Scope
The advance notice of importation process must be followed as per s. 9 of the Cosmetic Regulations and A.01.44 of the Food and Drug Regulations. It applies to:
- cosmetics requiring modification or relabelling prior to being sold as cosmetics in Canada
- drugs requiring modification or relabelling prior to being sold as cosmetics in Canada
- drugs requiring modification or relabelling prior to being sold as foods in Canada
- drugs that hold a valid Canadian market authorization (Drug Identification Number (DIN)/Homeopathic Medicine Number (DIN-HM)/ Natural Product Number (NPN)) and require modification or relabelling prior to being sold as drugs in Canada
Advance notice of importation
S.9 of the Cosmetic Regulations and A.01.044 of the Food and Drug Regulations require importers to provide Health Canada with advance notice of each proposed importation. Regulated parties may fill out the advance notice of importation form for multiple imports covering a 3-month period on a single form.
At the border
Upon arrival at the Canadian border, the Canada Border Services Agency (CBSA) refers cosmetics and drugs to Health Canada, who assess them to see if they are subject to s.9 of the CR and A.01.044 of the FDR.
Submitting an advance notice of importation form does not guarantee cosmetics and drugs entry into Canada. Health Canada will recommend refusal of cosmetics and drugs that do not meet all requirements. This includes:
- products that cannot be modified or relabeled to comply with regulatory requirements
- unauthorized drugs waiting for the issuance of a market authorization by Health Canada, including a:
- DIN
- NPN
- DIN-HM
- notice of compliance (NOC)
The future issuance of market authorizations is not considered to meet the modification or relabelling conditions of the Food and Drug Regulations.
Compliance and enforcement
Health Canada may conduct monitoring activities to measure industry’s compliance with the modification or relabelling requirement after import. Importers that do not comply with these requirements will be subject to compliance and enforcement action in accordance with:
- Compliance and Enforcement Policy for Health Products (POL-0001)
- Healthy Environments and Consumer Safety Branch Compliance and Enforcement Policy for cosmetics
Compliance and enforcement actions may include:
- refusal of future shipments
- stop-sale requests of non-compliant products
- requirement of advance notice for each individual importation of products that need relabelling or modification rather than 1 form covering a 3-month period
Contact us
Contact your nearest regional Health Canada office to:
- request a copy of the advance notice of importation form and instruction document
- ask questions about the advance notice of importation process
-
Atlantic
-
Quebec
-
Ontario
-
Manitoba-Saskatchewan
-
Alberta
-
British Columbia
E-mail: hc.bc.import.notice-avis.sc@canada.ca
Consumer Product Safety Program: 604-652-4240
Border Operations Program: 604-652-3829
Guidance Document
- Guidance Document
- Web Version/ Pdf Version
- Sunscreen Monograph
- Sunscreen Monograph
- Drug
- Food and Drug Regulations
- TPD – Therapeutic Products Directorate
- Food and Drug Regulations
- Natural Health Products
- Natural Health Product Regulations
- NHPD – Natural Health Products Directorate
- Natural Health Product Regulations
- Drug
- NNHPD – Natural and Non-prescription Health Products Directorate
- Old: NHPD – Natural Health Products Directorate
- US Food and Drug Administration
- Sunscreen Drug Products for Over-the-Counter Human Use
- Final Rule (2011)
- Sunscreen Drug Products for Over-the-Counter Human Use
Sunscreens as Natural Health Products
- Table 1
- Natural Health Products medicinal ingredients, source materials, and concentrations
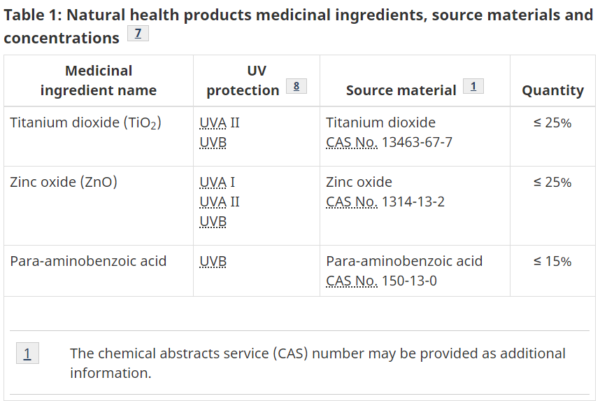
- NPN – Natural Product Number (if Sunscreens – NHPs)
- Labelling Requirement Checklist
Sunscreens as Drug
- Table 2
- Drug medicinal ingredients and concentration (monograph ingredients)
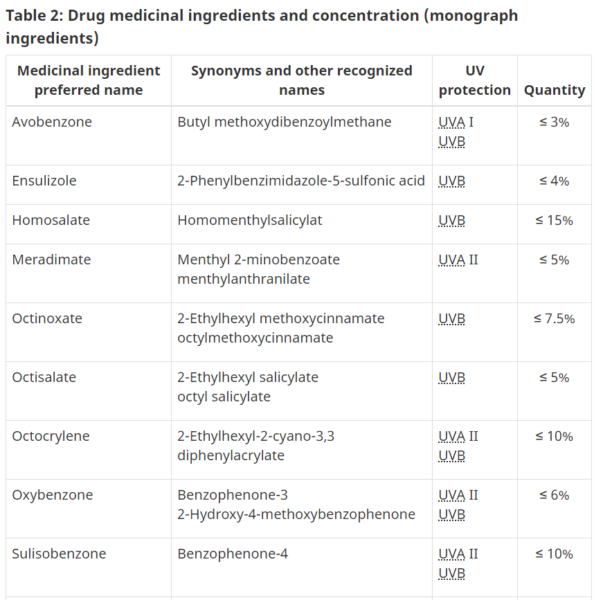
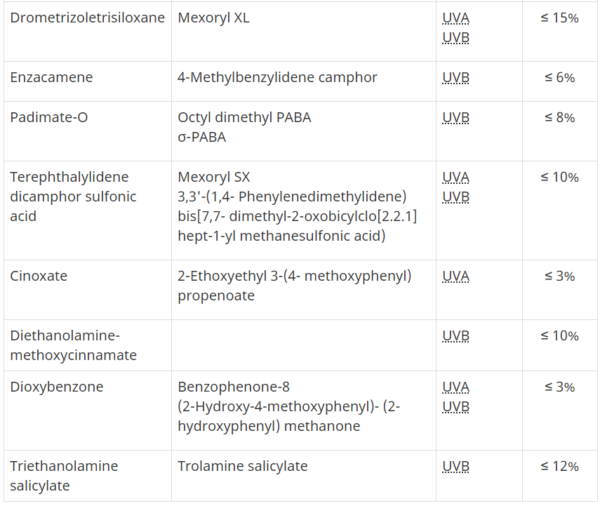
- DIN -Drug Identification Number (if Sunscreens – Drug)
- Applications and Submissions -Drug Products
Drug product labelling
- Food and Drugs Act and the Food and Drug Regulations
- Detail examples
- Brand name
- Drug Identification Number
- Lot #/expiry date
- Storage conditions
- Medicinal ingredient declaration
- Non-medicinal ingredients
- Proper or common name of the finished drug product
- Name and address of the Canadian DIN owner or..
- Flammability warning
- Pressurized container warning
- Net quantity declaration
- Detail examples
- Natural Health Product
- Natural Health Product Regulations
